Teranode's Real Time Intelligence (RTI) solution for Drug Development and Post-Market Safety is the only solution in the market that brings the analytical power of the a Big Data engine along with the flexible and rapid integration capability of a Business Process Automation platform. More specifically Teranode RTI provides:
|
|
| 1. |
Accurate, Tractable and Contextual Analysis of structured data and unstructured text from any number of internal or external sources, at every stage of the Drug Development Life Cycle |
|
|
| 2. |
Integrated social media listening platform |
|
|
| 3. |
Automatically review internal documents to detect and fix deviation from compliance and regulatory policies |
|
|
| 4. |
Monitor off label use related interactions, adverse events across drugs |
|
|
| 5. |
Seamless integration into your existing IT environment, with a case management engine to manage actions and alerts to draw attention to specific events and thresholds |
|
|
The underlying technology from RAGE Frameworks is in broad use across leading global corporations in multiple industries to solve highly complex aggregation, interpretation and analysis challenges, providing early warning and insights in areas such as investment research, enterprise risk, supplier risk and competitive intelligence, to name a few.” |
 |
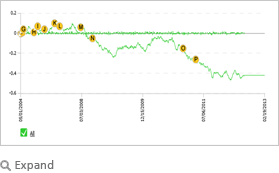
| This graph for Avandia illustrates the cumulative efficacy trend based on information from thousands of public and premium sources since 2004. The RAGE linguistics engine reviews and interprets unstructured text from any number of internal and external sources in real time, guided by a causal ontology, which in the case of Avandia includes therapeutic effects, side effects, adverse effects, label changes, FDA documents, off label use, tweets, major events, amongst others. Each article, news item, social media input contributes to a cumulative score which is represented by the graph to provide a comprehensive real time perspective on any FDA approved drug and others in the pipeline. |
|
|
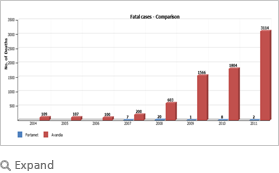
Insights at a glance
|
| |
|
|
|
